Title:
Investigation 11: What is the Rate Law of the Fading of
Crystal Violet Using Beer's Law?
Lab Partners:
Challenge or Problem Given: Prepare dilutions of a stock
Crystal Violet solution to construct a calibration curve for Crystal
Violet. Determine the rate of law for the fading of Crystal Violet when
reacted with Sodium Hydroxide, using absorbance at specific times and
calibration curve to determine concentration of Crystal Violet remaining
at those times.
MSDS
information: Include hazards and disposal for Crystal Violet and
Sodium Hydroxide
Procedure for Constructing a Calibration Curve for Crystal Violet: (be sure to include drawing of set up)
1. Connect a Colorimeter to Go Link! of the Vernier computer interface. Connect the Go Link to the chromebook using the proper cable.
2. Start the Graphical Analysis program on your chromebook. When program opens, absorbance should be displayed in lower right hand corner of screen
3. Calibrate the Colorimeter.
a. Place the blank of equal volumes
of water and 0.02 M NaOH in the cuvette slot of the Colorimeter and close the
lid.
b. Press the < or > button on the
Colorimeter to select the wavelength of 635 nm or 565 nm. Press the CAL button
until the red LED begins to flash and then release the CAL button. When the LED
stops flashing, the calibration is complete.
4. Using a serological pipet or measuring syringe for accuracy, prepare the series of standard dilutions of the crystal violet stock solution. Use the amounts calculated in the Pre-Lab assignment. Lab Hint: To avoid contaminating the stock solution, first use the pipet to add the required amount of distilled water to each test tube. Rinse the pipet three times with the stock solution, and then measure and add the required amount of stock solution to each test tube. Mix as needed.
5. Transfer the solutions to a cuvette and clean the outside with a lint free wipe. Place cuvette into the colorimeter and close the lid.
6. Measure and record the absorbance (A) of the stock solution and each standard solution (dilution) at the selected wavelength.
Procedure for Determining Rate Law for Fading Crystal Violet:
1. Connect a Colorimeter to Go Link! of the Vernier computer interface. Connect the Go Link to the chromebook using the proper cable.
2. Start the Graphical Analysis program on your chromebook. When program opens, absorbance should be displayed in lower right hand corner of screen. Press Ctrl D to open Data Collection Box. Change Length to 900 seconds and Sample Rate to 0.05 samples/second (20 seconds/sample).
3. Calibrate the Colorimeter.
a. Place the blank of equal volumes
of water and 0.02 M NaOH in the cuvette slot of the Colorimeter and close the
lid.
b. Press the < or > button on the
Colorimeter to select the wavelength of 635 nm or 565 nm. Press the CAL button
until the red LED begins to flash and then release the CAL button. When the LED
stops flashing, the calibration is complete.
4. Measure 10.0 mL of 25 μm crystal violet in a serological pipet and add it to a clean 50-mL beaker.
5. Rinse the pipet with distilled water several times and also with the sodium hydroxide solution. Measure 10.0 mL of 0.02 M sodium hydroxide in a serological pipet.
6. Add the sodium hydroxide into the 50-mL beaker with crystal violet. Mix and immediately press “Collect” to begin timing.
7. Transfer the reacting solutions to a cuvette and clean the outside with a lint free wipe. Place cuvette into the colorimeter and close the lid.
8. Watch as the computer records absorbance measurements every 20 seconds for 15 minutes.
Pre-lab questions: .
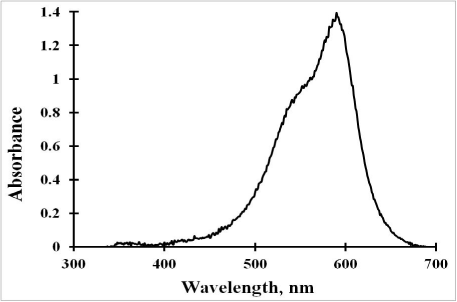
The visible absorption spectrum for crystal violet, CV+, is
shown in Figure 3. The concentration of the dye was 12.5 μM (12.5 × 10–6
M).

1. What would be the optimum wavelength for generating a Beer’s law calibration curve for crystal violet and measuring absorbance versus time for the reaction of CV+ with OH–? Explain your answer. Absorbance measurements are most accurate and sensitive in the range 0.2–1.0.
2. A calibration curve requires the use of several concentrations of the test solution. Using 25 μM CV solution as the stock solution, complete the following table to show how you would prepare 2.5, 5, 7.5, 10, and 12.5 μM solutions of CV+. Assume that the final solution volume should be 10.0 mL in all cases.
| CV Stock Solution | A | B | C | D | E | |
| Concentration (micromolar, μM) | 25 | 2.5 | 5 | 7.5 | 10 | 12.5 |
| Water (mL) | 0 | 5 | ||||
| Stock Solution (mL) | 10 | 5 |
3. Using your optimum
wavelength for the experiment, predict the estimated absorbance value for each
solution in Table 1. Record these values in the table. Hint: Keep in mind Beer’s
law from Equation 5 and the fact that the path length (b) and wavelength are
constant.
Data Tables:
1) Data table for Concentration of Crystal Violet and Absorbance
2) Data Table for Time, Absorbance, [CV+], ln [CV+], and 1/[CV+] (Can be done on Vernier and printed/pasted into lab book)
Analysis of Data:
1. Create a calibration curve for Crystal Violet by graphing absorbance vs concentration using data from first part of the lab.
2. Use the absorbance data collected at each time and the calibration curve to determine the concentration of CV+ at each time over the course of the rate trial. (Can show work for one and then plug equation into Vernier to have the program do the rest in data analysis)
3. Calculate the values of ln[CV+] and 1/[CV+] for each time. (Can show work for one and then plug equation into Vernier to have the program do the rest in data analysis)
4. Perform the graphical analysis determine the order of reaction for Crystal Violet (Can be done on Vernier and printed/pasted into lab book)
5. Write the rate law expression for the fading of Crystal Violet.
6. Determine the value of the pseudo-rate constant kʹ.
Conclusion:
State what was discovered through the investigation with supporting data and explain how that discovery addressed the challenge or problem given. Discuss precision of measuring tools and of data collection. Explain how what was learned in the investigation could be used in the real world. Discuss how the investigation could be improved or extended in the future.