AP BIO Lab 4: Photosynthesis
BACKGROUND
Photosynthesis
fuels ecosystems and replenishes the Earth’s atmosphere with oxygen.
The process of photosynthesis involves the use of light energy to convert carbon
dioxide and water into sugar, oxygen, and other organic compounds. This process
is often summarized by the following reaction:
6 H2O + 6 CO2 + light energy -->C6H12O6 + 6 O2
This process is an extremely complex one, occurring in two stages. The first stage, called the light reactions of photosynthesis, requires light energy. The products of the light reactions are then used to produce glucose from carbon dioxide and water. Because the reactions in the second stage do not require the direct use of light energy, they are called the dark reactions of photosynthesis. In the light reactions, electrons derived from water are “excited” (raised to higher energy levels) in several steps, called photosystems I and II. In both steps, chlorophyll absorbs light energy that is used to excite the electrons. Normally, these electrons are passed to a cytochrome-containing electron transport chain. In the first photosystem (PSII), these electrons are used to generate ATP. In the second photosystem (PSI), excited electrons are used to produce the reduced coenzyme nicotinamide adenine dinucleotide phosphate (NADPH). Both ATP and NADPH are then used in the dark reactions to produce glucose.
OBJECTIVES:
-Learn a technique to determine photosynthesis rates
-Compare photosynthesis rates under various conditions.
-Explain why the rate of photosynthesis varies under different environmental
conditions.
Photosynthesis/The Light Reaction
What could you measure to determine the rate of photosynthesis?
In this investigation, you will use a system that measures the accumulation of oxygen
.
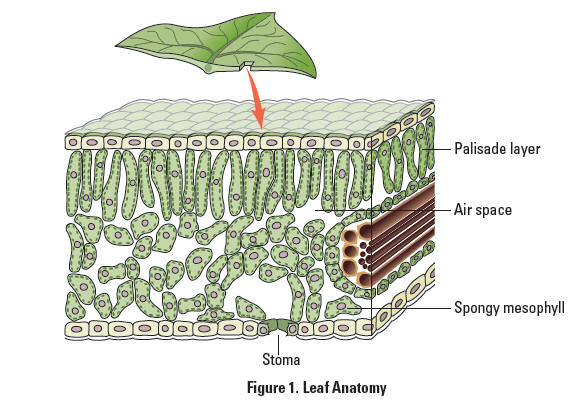
Because the spongy mesophyll layer of leaves (shown in Figure 1) is normally infused with gases (O
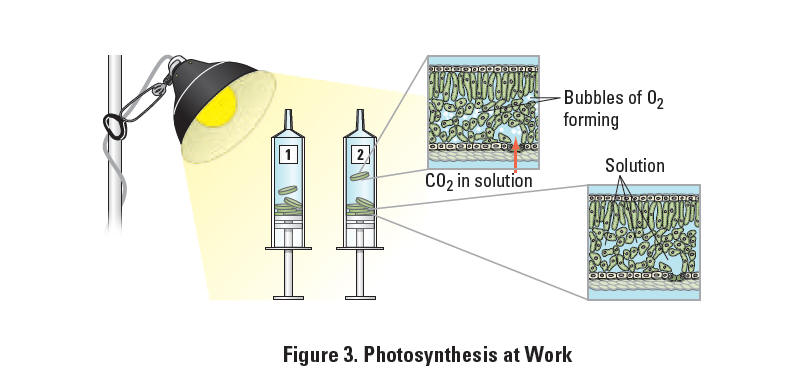
2 and CO2), leaves — or disks cut from leaves — normally float in water. What would you predict about the density of the leaf disk if the gases are drawn from the spongy mesophyll layer by using a vacuum and replaced with water? How will that affect whether or not the leaf floats? If the leaf disk is placed in a solution with an alternate source of carbon dioxide in the form of bicarbonate ions, then photosynthesis can occur in a sunken leaf disk. As photosynthesis proceeds, oxygen accumulates in the air spaces of the spongy mesophyll, and the leaf disk will once again become buoyant and rise in a column of water. Therefore, the rate of photosynthesis can be indirectly measured by the rate of rise of the leaf disks. However, there’s more going on in the leaf than that! You must also remember that cellular respiration is taking place at the same time as photosynthesis in plant leaves. (Remember that plant cells have mitochondria, too!) What else could be going on that might affect this process? Aerobic respiration will consume oxygen that has accumulated in spongy mesophyll. Consequently, the two processes counter each other with respect to the accumulation of oxygen in the air spaces of the spongy mesophyll. So now you have a more robust measurement tool —the buoyancy of the leaf disks is actually an indirect measurement of the net rate of photosynthesis occurring in the leaf tissue.When immersed in water, oxygen bubbles are usually trapped in the air spaces of the spongy mesophyll in the plant leaf. By creating a vacuum in this experimental procedure, the air bubbles can be drawn out of the spongy mesophyll, and the space is refilled by the surrounding solution. This allows the leaf disks to sink in the experimental solution. If the solution has bicarbonate ions and enough light, the leaf disk will begin to produce sugars and oxygen through the process of photosynthesis. Oxygen collects in the leaf as photosynthesis progresses, causing the leaf disks to float again. The length of time it takes for leaf disks to float again is a measure of the net rate of photosynthesis. This process is shown in Figure 3.
In other experiments, a blue dye (2,6-dichlorophenol-indophenol, or DPIP) can be used to replace NADPH in the light reactions. When the dye is oxidized, it is blue. When reduced, however, it turns colorless. Since DPIP replaces NADPH in the light reactions, when the light strikes the chloroplasts, electrons boosted to higher energy levels through photosynthesis will reduce DPIP and turn it from blue to colorless. Chloroplasts will be extracted from spinach leaves and incubated with DPIP in the presence of light. As the DPIP is reduced and becomes colorless, the resultant increase in light transmittance is measure over a period of time using a colorimeter.
PROCEDURE: (This procedure is to test effect of CO2 vs no CO2
in water on rate of photosynthesis... MODIFY the procedure for your purpose
question)
Step 1
Prepare 300 mL of 0.2% bicarbonate
solution for each experiment. The bicarbonate will serve as a source of carbon
dioxide for the leaf disks while they are in the solution.
Step 2
Pour the bicarbonate solution into a clear plastic cup to a depth of about 3 cm. Label this cup “With CO2.” Fill a second cup with only water to be used as a control group. Label this cup “Without CO2.” Throughout the rest of the procedure you will be preparing material for both cups, so do everything for both cups simultaneously.Step 3
Using a pipette, add one drop of a dilute liquid soap solution to the solution in each cup. It is critical to avoid suds. If either solution generates suds, then dilute it with more bicarbonate or water solution. The soap acts as a surfactant or “wetting agent” —it wets the hydrophobic surface of the leaf, allowing the solution to be drawn into the leaf and enabling the leaf disks to sink in the fluid.Step 4
Using a hole punch, cut 10 or more uniform leaf disks for each cup. Avoid major leaf veins. (The choice of plant material is perhaps the most critical aspect of this procedure. The leaf surface should be smooth and not too thick.)
Step 5
Draw the gases out of the spongy mesophyll tissue and infiltrate the leaves with the sodium bicarbonate solution by performing the following steps: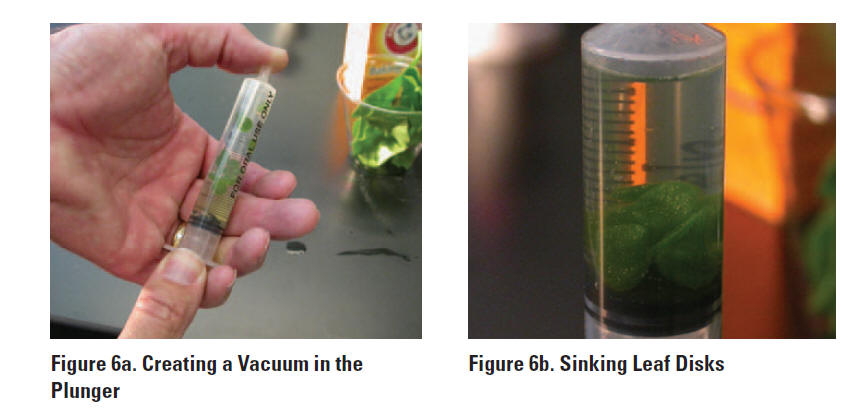
Step 6
Pour the disks and the solution from the syringe into the appropriate clear plastic cup. Disks infiltrated with the bicarbonate solution go in the “With CO2” cup, and disks infiltrated with the water go in the “Without CO2” cup.
Step 7
Place both cups under the light source and start the timer. At the end of each minute, record the number of floating disks. Then swirl the disks to dislodge any that stuck against the side of the cups. Continue until all of the disks are floating in the cup with the bicarbonate solution.Step 8
Record data in table and calculate rate of photosynthesis by dividing number of disks floating by time it took them to float.DATA TABLE:
| Independent Variable (unit) | Measurable Data (unit) | |||
| Trial 1 | Trial 2 | Trial 2 | Average | |
ANALYSIS:
1. Calculate your rate of photosynthesis or number of floating disks as a function of time for each of your trials. Calculate your average rate of photosynthesis for each test group.
2. Make a graph for your data. Be sure to use rate of photosynthesis calculated in #1 as your dependent variable.
3. What is the importance of establishing a control for this experiment?
4. Why must we consider respiration when performing this activity?
5. How do floating disks correspond to the rate of photosynthesis?
6. How does the baking soda solution affect photosynthetic rates?
7. Calculate chi squared or standard deviation analysis
CONCLUSION:
As usual from blue
sheet
.7